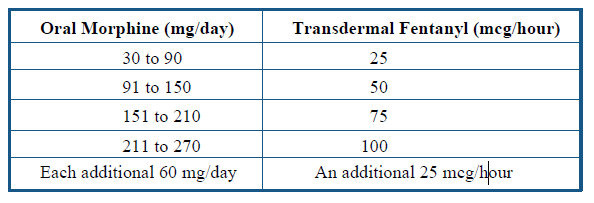
# Transdermal fentanyl and buprenorphine patches are prescribed in micrograms (mcg)/hour. Equivalent doses are based on the 24 hour dose of fentanyl or buprenorphine received from a patch. See product literature for further information. Based on buprenorphine to morphine ratio of 1:70-83. The manufacturers consider the initial dosages of transdermal fentanyl in Tables 8, 9, and 10 to be conservative estimates. 225 Do not use the dosage conversion guidelines in these tables to switch patients from fentanyl transdermal system to oral or parenteral opiates, since dosage of oral or parenteral opiates may be overestimated.
Associated Data
Abstract
Purpose
Transdermal fentanyl is effective for the treatment of moderate to severe cancer-related pain but is unsuitable for fast titration. In this setting, continuous subcutaneous fentanyl may be used. As data on the pharmacokinetics of continuous subcutaneous fentanyl are lacking, we studied the pharmacokinetics of subcutaneous and transdermal fentanyl. Furthermore, we evaluated rotations from the subcutaneous to the transdermal route.
Methods
Fifty-two patients treated with subcutaneous and/or transdermal fentanyl for moderate to severe cancer-related pain participated. A population pharmacokinetic model was developed and evaluated using non-linear mixed-effects modelling. For rotations from subcutaneous to transdermal fentanyl, a 1:1 dose conversion ratio was used while the subcutaneous infusion was continued for 12 h (with a 50 % tapering after 6 h). A 6-h scheme with 50 % tapering after 3 h was simulated using the final model.
Results
A one-compartment model with first-order elimination and separate first-order absorption processes for each route adequately described the data. The estimated apparent clearance of fentanyl was 49.6 L/h; the absorption rate constant for subcutaneous and transdermal fentanyl was 0.0358 and 0.0135 h−1, respectively. Moderate to large inter-individual and inter-occasion variability was found. Around rotation from subcutaneous to transdermal fentanyl, measured and simulated plasma fentanyl concentrations rose and increasing side effects were observed.
Conclusions
We describe the pharmacokinetics of subcutaneous and transdermal fentanyl in one patient cohort and report several findings that are relevant for clinical practice. Further research is warranted to study the optimal scheme for rotations from the subcutaneous to the transdermal route.
Electronic supplementary material
The online version of this article (doi:10.1007/s00228-015-2005-x) contains supplementary material, which is available to authorized users.
Introduction
For the treatment of moderate to severe cancer-related pain, strong opioids are the treatment of choice [1, ]. Fentanyl is a synthetic opioid with a high affinity for the μ-opioid receptor and is 75–100 times more potent than morphine [, ]. According to international guidelines, fentanyl is not the opioid of first choice [], but nonetheless, it is widely used for the treatment of cancer-related pain. Fentanyl is recommended in patients with renal failure []. Furthermore, because the incidence of constipation is lower in fentanyl compared to morphine [–] and it can be administered through a patch, it is a popular drug for the treatment of cancer-related pain. Fentanyl can also be used if an opioid rotation is necessary after failure on another type of opioid. Its low molecular weight and high lipid solubility make it suitable for transdermal delivery []. Although the first patches used a reservoir design carrying risks of drug leakage or abuse, currently available patches have a matrix design. They release fentanyl at a proposed rate of 12.5–100 μg/h and the amount delivered is proportional to the surface area of the patch. As a gradient is needed between the patch and the skin, the patch contains more fentanyl than is released. A mean bioavailability of 92 % (57–146 %) has been reported []. Reservoir and matrix patches and different types of matrix patches have been shown to have similar pharmacokinetic profiles [, ]. The slow decrease in fentanyl concentrations after transdermal patch removal and the delay before achieving the maximum plasma concentrations (both reflecting slow release of fentanyl) make transdermal fentanyl (patches) unsuitable for fast titration in patients with severe pain. In this setting, parenteral titration is therefore preferred. Subcutaneous administration has been proven to be safe and effective [, ] and has advantages over the intravenous route as no vascular access is needed, making it easier to change sites and avoiding complications associated with indwelling intravenous catheters. In addition, subcutaneous administration can also be applied safely in an out-of-hospital setting [].
In our cancer institute, patients with severe pain are preferably titrated with continuous subcutaneous opioids, and in this setting, fentanyl is frequently used. However, little is known about the pharmacokinetics of subcutaneously (sc) administered fentanyl as opposed to the transdermal (td) route. As part of a larger prospective pharmacologic opioid project, we studied the pharmacokinetics of fentanyl in hospitalized cancer patients with moderate to severe cancer-related pain. The purpose was to study the pharmacokinetics of fentanyl administered via the subcutaneous and transdermal routes to cancer patients. A second aim was to evaluate rotations from the subcutaneous to the transdermal route.
Patients, materials and methods
Between January 2010 and November 2013, patients admitted to the Erasmus MC Cancer Institute (Rotterdam, The Netherlands) and treated with fentanyl for moderate to severe cancer-related nociceptive pain were asked to participate in the study. Fentanyl Sandoz® Matrix patches were used in available doses of 12/25/50/75/100 μg/h and patches could be combined. Patches were applied to the chest wall or upper arm and were replaced every 72 h. The starting dose in opioid-naive patients was 12 μg/h and doses in other patients were based on previous treatment. In case of severe pain, patients were titrated by continuous sc infusion with the possibility of an extra bolus every hour. The dose of the bolus usually parallels the dose given per hour. Doses were titrated based on clinical effects. When pain control was reached and doses were stabilized, patients could be rotated to fentanyl (td) patches depending on the clinical setting. For the rotation of sc to td fentanyl, a 1:1 dose conversion ratio was used, based on data from previous studies [, ]. After applying the patch, the sc administration was continued in the same dose for 6 h, after which 50 % of the dose was given during an extra 6 h []. After 12 h of patch application, the sc administration was stopped. Patients treated with a patch were prescribed medication for the treatment of breakthrough pain, mostly oral morphine or oxycodone in an immediate release formulation but not rapid onset opioids. For all patients, co-medication was screened for the concurrent use of strong CYP3A4 inhibitors or inducers. Also, liver function was checked based on the laboratory values of bilirubin, alanine aminotranferase (ALT), aspartate aminotransferase (AST) and albumin. The study was approved by the medical ethics review board (MEC 09.332) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants. The trial was registered in the Dutch Trial Register (Trial registration ID: NTR4369, http://www.trialregister.nl/trialreg/admin/rctsearch.asp?Term=4369).
Pharmacokinetic sample collection
Patients were included in the study as soon as possible after admission to the ward or after the start of fentanyl. Blood samples for pharmacokinetic analysis were taken during a maximum of 72 h after the start of fentanyl and after each change in the opioid regimen (dose, route of administration). The protocol prescribed sampling twice a day, around 8 am and 8 pm, a baseline plasma sample before every change in the regimen and a series of samples maximally once a day around the administration of an extra subcutaneous bolus at baseline, 5, 15, 30 and 60 min after administration. Samples were collected using potassium EDTA tubes. After centrifugation of the tube, the supernatant was collected and stored at −70 °C until analysis at the laboratory of Translational Pharmacology (Erasmus MC Cancer Institute).
Measurements of fentanyl plasma concentrations
Fentanyl in plasma was quantitated using a validated UPLC-MS/MS method consisting of a Waters Acquity UPLC sample manager coupled to a triple quadruple mass spectrometer operating in the multiple reaction monitoring mode (MRM) with positive ion electrospray ionization (Waters, Etten-Leur, The Netherlands). The multiple reaction monitoring transitions was set at 337 → 188 for fentanyl and 342 → 188 for the internal standard fentanyl-d5.
Chromatographic separations were achieved on an Acquity UPLC® BEH C18 1.7 μm 2.1 × 100 mm column thermostated at T = 50 °C. A gradient at a flow rate of 0.350 mL/min was achieved with mobile phase A, composed of 2 mM ammonium formate and 0.1 % formic acid, and mobile phase B, composed of methanol with 0.1 % formic acid. A linear gradient was used, with 90 % mobile phase A from 0–0.50 min followed by 90–0 % mobile phase A, from 0.50 to 2 min, holding on 0 % mobile phase A (i.e. 100 % mobile phase B) for 2 min. This was succeeded by a linear gradient back to 90 % mobile phase A from 4.0 to 4.1 min, which was held for 1.9 min to re-equilibrate. The overall cycle time of the method was 6 min. The calibration curves were linear over the range of 0.100 to 10.0 ng/mL with the lower limit of quantitation validated at 0.100 ng/mL for fentanyl. The extraction of 200 μL of plasma involved a deproteinization step with 100 μL of internal standard solution in acetonitrile and 100 μL of acetone followed by a simple liquid–liquid extraction with 1-mL ethyl acetate after the addition of 100 μL of 4 % ammonium hydroxide. For fentanyl (linear calibration range 0.100–10.0 ng/mL), the within- and between-run precisions at five tested concentrations, including the lower limit of quantitation (LLQ), were ≤5.52 and ≤6.12 %, respectively, while the average accuracy ranged from 88.5 to 94.0 %. No adsorption of fentanyl was observed to the sampling and/or storing tubes. The inter-day coefficient of variation (CV) at five tested concentrations, including the LLQ, was ≤7.5 % in individual validation runs.
Population pharmacokinetic model for fentanyl
The analysis of log-transformed concentration–time data was carried out with non-linear mixed-effects modelling in NONMEM (version 7.3; Icon Development Solutions, Hanover, MD) by means of the first-order conditional estimation method with or without eta-epsilon interaction [18]. Model building was assisted by Perl-speaks-NONMEM (PsN version 4.2.0, http://psn.sourceforge.net/) [, ] and the graphical evaluation with R (version 3.0.3, http://www.r-project.org/) and Xpose (version 4.4.1, http://xpose.sourceforge.net/) [].
As a starting point, a one-compartment model with first-order absorption preceded by a lag time was used. Several model components were evaluated, including one- versus two-compartment disposition models, alternative absorption models following transdermal administration (first- versus zero-order), differences between the two administration routes in absorption parameters, i.e. absorption rate constant (ka) and lag time (tlag), and inclusion of allometrically scaled body weight on disposition parameters. Concentrations below the lower limit of quantification comprised less than 1 % of the data and were discarded from the analysis.
Inter-individual variability (IIV) in pharmacokinetic parameters was modelled using log-normal models. An occasion was defined as a transdermal dose followed by at least one observation, and inter-occasion variability (IOV) was evaluated on absorption parameters as proposed by Karlsson and Sheiner []:
where Pik represents the parameter P for the ith individual on occasion k, P is the typical parameter for the studied population, ηi is the patient-specific random effect describing the discrepancy between the typical and individual parameter and κik is the random effect accounting for the IOV. ηi and κik are assumed to be normally distributed with mean zero and estimated variance ω2 and π2, respectively.
Alternative residual error models were evaluated, including homoscedastic or heteroscedastic residual errors as well as a model combining both types of error.
Model evaluation
The selection between alternative models during the modelling process was based on scientific plausibility and statistical significance. Statistical evaluation comprised the analysis of goodness-of-fit plots, precision of parameter estimates, condition number and the likelihood ratio test based on the change of the objective function value (OFV). The OFV is given by minus twice the log likelihood, and a difference in OFV (ΔOFV) between nested models is approximately χ2 distributed. A ΔOFV of 3.84, 6.64 and 10.8 corresponds to p values of 0.05, 0.01 and 0.001, respectively, when one parameter is added to the model (1 df). The Akaike information criterion (AIC) was used to compare non-hierarchical models. The magnitude of η- and ε- shrinkage was computed according to Karlsson and Savic [] to judge the reliability of various diagnostic plots. The uncertainty of parameter estimates was assessed using the non-parametric bootstrap procedure in PsN (1000 bootstrap datasets). The predictive performance of the final model was evaluated with a population prediction-corrected visual predictive check (pcVPC) through 1000 simulations of the dataset [].
Results
Patients
Plasma samples for pharmacokinetic analysis were available for 52 patients (Table (Table1).1). Three patients participated in the study twice. Treatment with td and sc fentanyl in relation to the observations for all patients is shown in supplemental figure 1. In 13 patients, samples were available during sc treatment without previous td administration; in 9 patients, samples were available during treatment with td fentanyl without previous or concurrent sc treatment; and in 32 patients, samples were available during treatment with sc or td fentanyl, but the other treatment route was given until shortly before sampling (semi-simultaneous treatment) or simultaneously. The majority of patients (n = 33) already used transdermal fentanyl before admission. In total, 942 fentanyl plasma samples were available with a median of 15 sparse samples per patient (range 1–86) and a median concentration of 1.33 ng/mL (range 0.122–10.7 ng/mL). One patient used a strong CYP3A4 inducer—carbamazepine 200 mg—during his study period. In none of the patients, the combination of AST and/or ALT above upper limit of normal (ULN), bilirubin above ULN and albumin below lower limit of normal was found, and therefore it was concluded that none of the patients had liver failure. Doses for the transdermal route varied from 12 to 400 μg/h (median 50 μg/h), and doses for the continuous subcutaneous infusion ranged from 10 to 300 μg/h (median 75 μg/h).
Table 1
| Characteristics (n = 52) | No. (%) |
|---|---|
| Median age (years)—range | 63 (23–80) |
| Sex | |
| Male | 33 (63) |
| Female | 19 (37) |
| Race | |
| Caucasian | 47 (90) |
| Other | 1 (2) |
| Unknown | 4 (8) |
| WHO performance status | |
| 0 | 0 |
| 1 | 19 (37) |
| 2 | 17 (33) |
| 3 | 4 (8) |
| Unknown | 12 (23) |
| Median body mass index—range | 25 (18–40) |
| Median NRS in rest at start of fentanyl or on admission—range | 5 (2–10) |
| Primary tumour localization | |
| Breast | 8 (15) |
| Colorectal | 5 (10) |
| Prostate | 7 (13) |
| Soft tissue sarcoma/GIST | 6 (12) |
| Urinary tract (including the kidney) | 8 (15) |
| Other | 18 (35) |
| Median albumin—range | 39 (29–49) |
| Median AST (U/l)—range | 31 (13–216) |
| Median ALT (U/l)—range | 22 (7–131) |
| Median total bilirubin (μmol/L)—range | 7 (3–16) |
Fentanyl pharmacokinetics
The pharmacokinetics of fentanyl-administered sc and td were best described by a one-compartment model with first-order elimination and separate first-order absorption processes for each route. The residual error was most adequately described by a heteroscedastic model parameterised as an additive model on the log-scale. Due to the sparse sampling design, we were unable to estimate all model parameters satisfactorily, particularly with respect to parameters describing the absorption part. Hence, the apparent volume of distribution (V/F) was fixed to 280 L []. A sensitivity analysis carried out with values of V/F ±50 % fixed in 10 % increments showed the model to be insensitive to the value and other parameter estimates to be stable within the tested range, with only tlag and ka,sc varying slightly (less than ±25 % deviation from the final PK parameter values). Inclusion of allometrically scaled body weight on CL/F and V/F was found to explain some variability and was kept to increase model stability. The final population model parameters including bootstrap results are presented in Table Table22.
Table 2
Typical population pharmacokinetic parameter estimates for subcutaneous and transdermal fentanyl and bootstrap analysis results
| Parameter (units) | NONMEM estimate (%RSE)a | Bootstrap mean (95 % CI)b | ||
|---|---|---|---|---|
| Structural model parameters | ||||
| ka subcutaneous (h−1) | 0.0358 | (24.4) | 0.0374 | (0.0248, 0.0555) |
| tlag transdermal (h) | 4.73 | (21.2) | 4.65 | (2.25, 6.98) |
| ka transdermal (h−1) | 0.0135 | (16.8) | 0.0140 | (0.0105, 0.0188) |
| V70kg/F (L)c | 280 | (fix) | – | |
| CL70kg/F (L h−1)d | 49.6 | (9.36) | 50.4 | (40.9, 61.6) |
| Inter-individual variability (%CV) | ||||
| ka subcutaneous | 93.5 | (15.2e) | 91.1 | (59.6, 119) |
| F transdermal | 42.3 | (30.0e) | 45.7 | (19.7, 67.8) |
| ka transdermal | 42.4 | (23.9e) | 41.4 | (10.5, 59.2) |
| CL/F | 43.2 | (15.2e) | 41.6 | (27.1, 53.9) |
| Inter-occasion variability (%CV) | ||||
| ka transdermal | 32.8 | (51.1e) | 39.2 | (12.0, 77.0) |
| Residual unexplained variability (%CV) | ||||
| Proportional residual error | 23.4 | (5.17f) | 23.2 | (20.6, 25.6) |
aThe condition number of the final model was 24.99
bMean and 95 % bootstrap percentile confidence intervals. Runs with estimates near a boundary (n = 150), rounding errors (n = 165) or crashed (n = 3) were skipped when calculating results
cV70kg/F = 280 × (WT/70)
dCL70kg/F = estimate × (WT/70)0.75
e%RSE is reported on the approximate standard deviation scale (standard error/variance estimate)/2. η-shrinkage for inter-subject variability ranged between 14.6 and 48.4 % and η-shrinkage for inter-occasion variability was >35 %
fε-shrinkage was 5.97 %
CI confidence interval; CL70kg/F apparent clearance for a subject with 70 kg; %CV percent coefficient of variation, reported as sqrt(variance) × 100 %; F bioavailability; ka absorption rate constant; %RSE relative standard error; tlag absorption lag time; V70kg/F apparent volume of distribution for a subject with 70 kg; WT weight (kg)
The estimated population value for CL/F in a 70-kg subject was 49.6 L/h. The estimation of a tlag for td administration led to an improvement of the model fit (p value <0.001) with the final value of 4.73 h. In contrast, the inclusion of a tlag was not relevant for sc administration. The model was compared with a model with zero-order absorption for td fentanyl, and the AIC was clearly in favour of the first-order absorption (AIC more than 60 points lower). The estimated absorption rate constant for subcutaneous fentanyl was 0.0358 h−1 and for transdermal fentanyl 0.0135 h−1.
IIV was included on ka for both routes (93.5 and 42.4 % for sc and td, respectively), td bioavailability and apparent clearance (CL/F). Bioavailability of td fentanyl was allowed to differ between individuals with an estimated variability of 42.3 %. IOV on td ka resulted in a significant improvement of the model (p < 0.01) with an estimated value of 32.8 %. The consequence for rate and extent of absorption following td administration, given these characteristics, is illustrated in Fig. 1.
Stochastic simulation of fentanyl plasma concentrations versus time after application of a transdermal patch with a delivery rate of 50 μg/h in 52 patients
The model was found to describe the observed concentrations well (Fig. 2). The performance of the model to predict median concentrations was good as illustrated by a pcVPC shown in Fig. 3. Additional goodness-of-fit plots can be found in Supplemental data.
Goodness-of-fit plots for the final model. Observed fentanyl plasma concentrations versus population predictions (left panels) and individual predictions (right panels) in normal (top panels) and logarithmic scale (bottom panels). The solid line represents the line of identity (x = y) and the dashed line represents a linear regression line
Population prediction-corrected visual predictive check for the final model for subcutaneous and transdermal fentanyl. The x-axis represents the time after the first recorded dose of fentanyl after admission. Dots are the population predicted-corrected individual observations, and the solid and dashed lines represent the median and the 10th and 90th percentiles of the observed data, respectively. The shaded areas represent the simulation-based 95 % confidence interval for the simulated data percentiles
Evaluation of rotations from subcutaneous to transdermal fentanyl
For 14 patients, multiple plasma samples were available shortly before and after rotation from sc to td fentanyl using the 12 h scheme. In 12 of these patients, a rise in plasma fentanyl concentrations was seen after application of the first patch. Furthermore, the intensity of side effects increased in 9 patients while in 3 patients, severe fentanyl-related toxicity occurred, necessitating adjustment of treatment. The severe toxicity consisted of respiratory depression, severe drowsiness and nausea.
By using the final model, fentanyl plasma concentrations expected around and after rotation were predicted for a population of 52 patients through stochastic simulation. Figure Figure44 illustrates plasma fentanyl concentrations during the rotation from a sc infusion of 50 μg/h to a td patch with a delivery rate of 50 μg/h using the 12-h scheme. After the application of the td patch, the simulated median peak concentration is higher than the steady-state concentration of subcutaneous fentanyl. In addition, concentrations immediately after the end of the rotation scheme, i.e. 12 h after the application of the patch, are very variable with the 10th and 90th percentiles equal to 0.87 and 3.22 ng/mL (median value 1.68 ng/mL). Simulated fentanyl plasma concentrations using a 6-h scheme [] produced similar results, and comparative plots can be found in Supplemental data.
Simulated fentanyl plasma concentrations during the rotation from a subcutaneous infusion of 50 μg/h at steady state to a transdermal patch with a delivery rate of 50 μg/h using the 12-h scheme (1000 simulations of 52 subjects). Following this scheme, the subcutaneous administration is continued in the same dose for 6 h after applying the transdermal patch, after which 50 % of the dose is given during an extra 6 h. The simulated solid line represents the median of the simulated data, and the shaded area represents the 80 % prediction interval. The vertical dashed line represents the time of patch application
Discussion
This prospective study in Caucasian cancer patients treated with fentanyl provides us with new insights into the pharmacokinetics of fentanyl which are relevant for clinical practice.
Firstly, we developed a population pharmacokinetic model for sc and td fentanyl from a high number of sparse samples in this patient cohort. We found that a one-compartment model adequately describes the pharmacokinetics of sc and td fentanyl, similarly to the results of previous studies with td fentanyl [, ]. We were able to distinguish inter-individual variability between absorption and elimination pharmacokinetic parameters that along with inter-occasion and residual variability explain the high variability in plasma concentrations and possibly also clinical effects.
Similar PK models have been described following td administration previously [, ]. In our study, the CL/F was estimated to 49.6 L/h, which is similar to the values of 40.8 and 42.4 L/h obtained in previous PK studies [, ]. Furthermore, in line with previous models, the absorption from td patches over 72 h was found to be closer to a first-order than to a zero-order process, with a potential to lead to fluctuations in plasma concentrations during treatment. Indeed, fluctuation in plasma concentrations has been reported in several studies [–]; however, the clinical relevance of this finding was never widely acknowledged. In clinical practice, however, many patients report either lower pain scores and/or more side effects after patch change, and on the other hand, for worsening of pain during the third day, a patch is used [, ].
The estimated absorption rate constant and absorption lag time are in agreement with the values found by Bista et al. [] (0.013 h−1, ka) and Kokubun et al. [] (0.0145 h−1 and 4.93, ka and lag time) for td fentanyl. Such slow absorption relative to elimination (absorption and elimination half-lives 51.3 and 3.91 h, respectively) results in that the decline in plasma concentrations after achieving the peak following transdermal administration reflects absorption rather than elimination. The Tmax predicted by our model in a typical patient was about 20.5 h after the administration of a patch. This value is known to vary substantially between patients and values in the range 12–48 h have been reported []. The td absorption with large variability is illustrated in Fig. 1.
For sc fentanyl, published PK data are limited. In the only other study in patients treated with continuous infusion of sc fentanyl, only one plasma sample was taken showing considerable variability, but no PK parameters were presented []. Capper et al. [] described the pharmacokinetics of fentanyl after a bolus of 200 μg fentanyl sc in nine healthy volunteers and reported a CL/F of 53.7 L/h, similar to our estimate, and a rapid absorption (Tmax 10–30 min). We found a slow absorption with substantial IIV in a situation in which fentanyl dosages were titrated using continuous infusion with extra boluses as needed for pain control. The estimation of a separate ka following sc boluses was tested but not supported by the data. In addition, the model was evaluated with a fast absorption process following sc administration by fixing ka for this route (2 h−1). However, goodness-of-fit plots and the fit of the model was statistically significantly worse (p < 0.001). In four patients in our study, plasma samples were available after stopping sc fentanyl because of rotation to another type of opioid. In all, a slow decrease in fentanyl plasma concentrations was noticeable which supports our data. It may be that also after subcutaneous treatment, some subcutaneous dose depot is formed, as has been reported for td fentanyl [], but there are no firm data following sc infusion. Thus, our model describes sc infusion data, but mechanistic conclusions should not be drawn. However, if the slow absorption would be that slow, it suggests that continuous fentanyl is less suitable for fast titration.
High to moderate variability in PK parameters and plasma concentrations has been reported before for td fentanyl, but literature on sc fentanyl is scarce. Kokubun et al. and Bista et al. [] estimated moderate IIV on CL/F to 43.5 and 38.5 %, respectively, following td patches. Although there are differences in patch type (reservoir versus matrix) and study populations, i.e. regarding the amount of sc fat/body mass index and hepatic metabolism, IIV was in agreement with our estimate of 43.2 %. The IIV on ka in the study of Kokubun was substantially greater (71.9 %) than the 42.4 % we obtained, but we also found different occasions as a significant source of variability (IOV, 32.8 %). Other studies have reported substantial variation in bioavailability (range 60 to 97 %), in the measured rate of absorption (e.g. 12.5 to 60.4 μg/h with a patch of 50 μg/h) [] and in inter- and intra-subject variability in plasma fentanyl concentrations (50.7 and 34.4 %, respectively) [].
Lastly, this is to our knowledge the first evaluation of rotations from sc to td fentanyl, using the scheme described by Kornick et al. [] who studied rotations from the intravenous (iv) to the transdermal route. More recently, a scheme using a two-step taper of iv fentanyl in 6 h was found to be safer than the 12-h method []. In a PK study by the same group, using the 6-h scheme, a rise in plasma concentrations was seen after 3 h but without adverse effects []. According to the current study, the use of the 12-h scheme and a 1:1 dose conversion may lead to a rather steep rise in plasma concentrations for some patients and clinically evident toxicity. Based on the final model, we simulated rotations using the 6-h scheme. This scheme may also lead to a rise in plasma levels and therefore potential toxicity. This is probably caused by the fact that plasma concentrations fall slower after stopping a sc administration than after an iv administration and by the finding that absorption following td administration appears to follow a first-order process. For confirmation of our findings, we have planned a prospective pharmacokinetic evaluation study of different rotation schemes without overlap of routes and with or without dose reduction of the first patch.
Strengths of our study are the longitudinal data that we assembled in one patient cohort and the large number of samples available for PK analysis. One limitation in our study was that, although we were able to estimate IIV and IOV variability in PK parameters, due to a limited sample size, we did not investigate possible sources of variability through covariate modelling. Furthermore, due to semi-simultaneous administration following different routes of administration, the observed concentrations were the sum of those obtained following each route. Especially, many patients started on sc fentanyl after hospital admission while they already used fentanyl td at home, and sc bolus injections for rescue were frequently administered over the full study period. Although the semi-simultaneous administration was accounted for in modelling, the study design was not optimal for modelling purposes.
In conclusion, this study describes the pharmacokinetics of sc and td fentanyl in one patient cohort. Findings relevant for clinical practice are the moderate to large IIV and IOV and that absorption following td administration potentially may lead to fluctuations in plasma concentrations. Furthermore, published rotation schemes for rotations from intravenous to transdermal fentanyl might not be applicable on rotations from subcutaneous to transdermal fentanyl.
Electronic supplementary material
Fig. 1(219K, jpg)Treatment with transdermal and subcutaneous fentanyl in relation to the observations for all patients. (JPG 218 kb)
Fig. 2(80K, jpg)Additional goodness-of-fit plots for the final model. Conditional weighted residuals versus time after the first recorded dose of fentanyl following admission (upper left panel), conditional weighted residuals versus population predictions (upper right panel) and absolute individual weighted residuals versus individual predictions (lower left panel). The grey line is a tendency line. (JPG 79 kb)
Fig. 3(9.6K, pdf)Simulated fentanyl plasma concentrations during the rotation from a subcutaneous (sc) infusion of 50 μg/h at steady-state to a transdermal (td) patch with a delivery rate of 50 μg/h using the 12-h scheme (left panel) and the 6-h scheme (right panel). In the 12-h scheme, the sc administration is continued in the same dose for 6 h after applying the td patch, after which 50 % of the dose is given during an extra 6 h. In the 6-h scheme, the sc administration is continued in the same dose for 3 h after applying the td patch, after which 50 % of the dose is given during an extra 3 h. The vertical dashed lines represent the start and the end of the rotation scheme. The simulated solid line represents the median of the simulated data and the shaded area represents the 80 % prediction interval (1,000 simulations of 52 subjects). (PDF 9 kb)
Contribution of authors statement
AO, PB, CR and RM designed the study. AO, EK, CR and RM performed research for the study. AO, JA, SJ, PB and AF analyzed the data obtained. JA, SJ and AF contributed new methods or models. All authors wrote the paper.
Compliance with ethical standards
The study was approved by the medical ethics review board (MEC 09.332) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Disclosures
This study was financially supported by the Netherlands Organisation for Health Research and Development (ZonMw project number 1151.0014), Cornelis Vrolijk Fund and Stichting Voorzieningenfonds Palliatieve Zorg Dirksland.
Conflicts of interest
The authors declare that they have no competing interests.
Footnotes
Astrid W. Oosten and João A. Abrantes contributed equally to this work.
References

Associated Data
Abstract
Background
The effectiveness and safety of switch from oral oxycodone to fentanyl patch is little known. Here, we investigated if early phase opioid switch from low dose of oral oxycodone to transdermal fentanyl matrix patch provided any benefits for patients with thoracic malignancy and stable cancer-related pain.
Methods
This open-label two-centered prospective study enrolled patients with thoracic malignancy suffering persistent malignancy-related pain with numeric rating scale of pain intensity ≤ 3 which had been controlled by oral oxycodone ≤ 20 mg/day. Eligible patients switched from oral oxycodone to 12.5 μg/h of transdermal fentanyl matrix patch. The dose was allowed to be titrated upwards every 3 day by 25-50%, except for the first increase from 12.5 μg/hr to 25 μg/hr,until achieving adequate pain control. The data on patients’ global assessment scores measured on a five-step scale, an 11-point numeric rating scale of pain intensity, the severity of adverse effects using a four-point categorical rating scale, and the Epworth sleepiness scale questionnaire were collected for 15 days.
Results
Forty-nine eligible patients were analyzed. Overall patients’ satisfaction score significantly improved from day 1 (2.7 ± 0.9) to day 15 (2.3 ± 0.9) (p < 0.05), and 90% and 78% of patients remained to receive the minimum dose of fentanyl patch on day 8 and 15 from the opioid switch. There was a significant difference in sleepiness throughout the study period, though no difference was detected in pain intensity and other adverse effects.
Conclusion
Transdermal fentanyl matrix patch is an alternative analgesic option for a stable cancer pain in patients with thoracic malignancies.
Background
Cancer-related pain is the most important concern in patients suffering cancer. More than 80% of patients with advanced disease experience cancer-related pain caused largely by direct tumor invasion []. Cancer-related pain often disturbs activities of daily living and deteriorates quality-of-life [].
Opioids are the mainstay of analgesic therapy for moderate to severe cancer-related pain, according to the current guidelines [,]. Oxycodone, a semi-synthetic opioid analgesic, has become a cornerstone in symptom management of cancer-related pain [-]. Controlled-release of oxycodone is often administered as the first-line strong opioid, typically at an initial dose of 5-10 mg every 12 hours in opioid-naïve patients, when non-steroidal anti-inflammatory drugs (NSAIDs) and weak opioids are ineffective.
On the other hand, the high lipid solubility and low molecular weight of fentanyl is suitable for transdermal administration. In 2002, a transdermal therapeutic system (TTS) for fentanyl incorporating a gel reservoir technology was first marketed as the Durotep®Patch (Janssen-Pharma, Japan). In 2008, a transdermal fentanyl matrix patch (Durotep®MT patch, Janssen-Pharma, Japan), a new TTS that contains fentanyl dissolved in the adhesion layer, has become available in Japan. The smallest size of the matrix patch, with fentanyl release rates of 12.5 μg/h, makes it possible to switch from lower dose of other opioid and then gradually titrate to the effectual dose []. Fentanyl may has not only a powerful analgesic effect, but advantage of less frequent and milder adverse effects such as nausea, vomiting, constipation and sleepiness [-]. Compared with oral morphine, fentanyl may have advantage especially in constipation, but there are concerns over methodological problems of constipation assessment. In a crossover trial, constipation was less frequent under transdermal fentanyl than under oral morphine, though the assessment methods of constipation remained unclear []. In another comparison study, the use of laxatives given by nurses was investigated instead of assessment of constipation and shown to be less frequent in fentanyl []. Two meta-analyses [,] and a systematic review [] also indicated a significant reduction in constipation for fentanyl patch compared with oral morphine. In contrast, another prospective comparison study showed that transdermal opioids of fentanyl and buprenorphine had no benefit over controlled release oral hydromorphone in gastrointestinal symptoms including constipation []. However, the evidence on an advantage of fentanyl patch over oral opioids is low level, because the trials regarding fentanyl patch were limited, not blinded and of low methodological quality [].
Opioid switch is a therapeutic maneuver aiming at improvement of analgesic response and reduction of adverse effects []. The maneuver includes change to different medication using the same administration route, alteration of administration route maintaining the current medication, or both. Fentanyl patch is the most favorable candidate of opioid switch from oral opioids because of less adverse effects and easier administration route [,]. Switch from oral morphine to transdermal fentanyl patch was shown as an effective and safe method [,], though the drop-out rate with fentanyl was higher than that with morphine in a crossover trial []. In contrast, little is known about the benefits of switch from oral oxycodone to fentanyl patch.
In this study, we investigated if early phase opioid switch from low dose of oral oxycodone to transdermal fentanyl matrix patch provided any benefits for patients with stable cancer-related pain.
Methods
Patient eligibility
Patients with thoracic malignancy-related pain were recruited in Osaka Police Hospital and Osaka University Hospital. The patients who met all the following criteria were eligible; 1) confirmed diagnosis of thoracic malignancy, 2) age ≥ 20 years, 3) persistent malignancy-related pain refractory to non-steroidal anti-inflammatory drugs (NSAIDs) or acetaminophen, 4) numeric rating scale (NRS) for pain ≤ 3 for at least two consecutive days by low dose (20 mg/day or less) of oral oxycodone (Oxycontine®, Shionogi-Pharma, Japan). Exclusion criteria were as follows; 1) prior history of allergy or hypersensitivity to opioids, 2) prior history of opioid abuse, 3) clinically significant cardiac, renal or hepatic insufficiency, 4) increased intracranial pressure or impaired cognitive function, 5) active and extensive skin disease precluding application of the transdermal delivery system, 6) persistent fever of 40 degrees Celsius or over, 7) pregnancy, lactation and suspicion of being pregnant, 8) prior use of any opioid-antagonists within 2 days before switching to fentanyl patch. Concomitant treatments such as chemotherapy and radiotherapy at the enrollment were accruable.
Study design and treatment
All patients recorded the following data daily for 15 days of fentanyl patch treatment; 1) pain intensity using an 11-point NRS (scales 0–10), 2) the severity of adverse effects (e.g., nausea, vomiting, constipation and sleepiness) using a four-point categorical rating scale (none = 0, not hard = 1, hard but endurable = 2 and unendurably hard = 3), 3) the number of defecation per day, 4) the Epworth sleepiness scale (ESS) questionnaire except for the question on sleepiness during driving a car because strong opioids are not permitted for drivers in Japan, 5) the frequencies of immediate-release oxycodone (Oxinorm®, Shionogi-Pharma, Japan) medications per day. Time of recording diary was arbitrary. The diaries were collected after 15 days of fentanyl patch treatment. The patients' global assessment scores were also rated using a five-step scale (very satisfied = category 1, satisfied = 2, neither satisfied nor dissatisfied = 3, dissatisfied = 4 and very dissatisfied = 5) [] on days 1, 8 and 15. Oral oxycodone was switched to 12.5 μg/h of transdermal fentanyl matrix patch. In the morning on the switch day (day 1), a fentanyl patch (2.1 mg/3 days) was applied at the same time of the oral intake of the last controlled-release oxycodone dose. Rescue immediate-release oxycodone dose to relieve the breakthrough pain was adjusted according to the dose of transdermal fentanyl patch. The dose of transdermal fentanyl patch was allowed to be titrated upwards every 3 days by 25-50%, except for the first increase from 2.1 mg/patch (12.5 μg/hr) to 4.2 mg/patch (25 μg/hr), until patients could achieve adequate pain control. Transdermal fentanyl patch treatment was continued for 15 days (5 replacements). Neither addition nor dose change of other supplementary analgesics was permitted during the study.
The primary endpoint in this study was patients' global assessment scores. Secondary endpoints were pain intensity, adverse effects and use of rescue oxycodone.
The study protocol was approved by the Institutional Review Board of Osaka University Hospital and the Osaka Police Hospital ethics committee, adhered to the principles outlined in the Guideline for Good Clinical Practice (January 1997) and Declaration of Helsinki (1996), and registered as UMIN000011067. Written informed consent was obtained from all patients before commencement of the study.
Statistical analysis
We expected that 85% of patients would give the global assessment scores 1 to 3, on the basis of the data from a previous Japanese clinical trial of Durotep®MT patch reporting that the patients’ satisfaction was 89.4% []. The estimated number of patients for analysis was 49 with a confidence interval of 95% and β-error of 10%. Given the possibility of deviation from assessment, 60 patients were necessary.
Listwise deletion was adopted in cases in which we missed data at opioid switch (day 1). Patients who dropped out without completion of 15-day patch treatment were also deleted from analysis. Missing data at days 8 and 15 from opioid switch were handled using last observation carried forward (LOCF) imputation technique.
The data for normally distributed continuous variables, discrete variables, and categorical variables are expressed as the mean ± standard deviation (SD), median with range, and frequency. Friedman test, with a post-hoc Steel test, was used to compare longitudinal changes in numeric and categorical rating scales and ESS questionnaire scores. Significance was set at p < 0.05. All statistical analyses were performed using Statcel statistical package (Statcel3; OMS Inc., Tokorozawa, Japan).
Results
Although enrollment to this study was planned to complete within 2 years, only 50 patients were accrued for 4 years, despite of 2-year extension of the study period. Therefore, this study was terminated in December 2012 because of delayed accrual. The final accrual did not reach 60 patients, the preplanned sample size.
From February 2009 to December 2012, a total of 50 eligible patients switched opioids. Almost all patients completed 15 days of fentanyl matrix patch treatment except one patient who suddenly died of cancer progression on the 13rd day from opioid switch. During the study period, 51% and 24% of patients concomitantly received chemotherapy and radiotherapy, respectively. The baseline characteristics are shown in Table 1.
Table 1

| Sex | |
| Male/Female | 43/6 |
| Age (years-old) | 69.0 ± 6.9 |
| Body mass index (kg/m2) | 20.4 ± 4.9 |
| Cancer (Histology) | |
| Lung Cancer (Ad/SQ/SCLC/Others) | 48 (26/14/4/4) |
| Mesothelioma | 1 |
| Stage | |
| IIB/IIIA/IIIB/IVa | 1/4/7/37a |
| ECOG PS | |
| 0-1/2/3/4 | 28/13/5/3 |
| Pain locationb | |
| Neck-shoulder/Upper limb/Chest/Hypochondrium/Lower back/Glutaeus to thigh | |
| 4/2/32/1/7/5 | |
| Pain causesb | |
| Distant metastasis (bone)/Invasion/Pleural dissemination | |
| 22 (17)/18/11 | |
| Duration of cancer pain (months) | |
| mean ± SD | 4.3 ± 4.4 |
| median (range) | 2 (0.27–18) |
| Duration of controlled-release oxycodone administration (months) | |
| mean ± SD | 2.0 ± 4.3 |
| median (range) | 0.6 (0.1 - 19.7) |
| Fentanyl patch treatment site | |
| day 1 | |
| Hospitalized/Outpatient | 40/9 |
| day 8 | |
| Hospitalized/Outpatient | 32/17 |
| day 15 | |
| Hospitalized/Outpatient | 22/27 |
| Concomitant systemic chemotherapy | |
| Yes/No | 25/24 |
| Concomitant radiotherapy | |
| Yes/No | 12/37 |
Ad, Adenocarcinoma; SQ, Squamous cell carcinoma; SCLC, Small cell lung carcinoma; ECOG PS, European Clinical Oncology Group Performance status; SD, standard deviation.
aincluding a case with malignant mesothelioma in c-stage IV.
bThere were two overlapping.
Overall patients’ satisfaction was significantly different throughout the study period (Friedman test; p < 0.01). The proportion of patients in category 1 or 2 (‘very satisfied’ or ‘satisfied’) for the patient's global assessment score increased from 43% on day 1 to 63% on day 8 and 61% on day 15, while the proportion of patients in category 1–3 (‘very satisfied’, ‘satisfied’ or ‘neither satisfied nor dissatisfied’) hardly changed from 87% on day 1 to 91% on day 15 (Table 2).
Table 2
Change of patients’ global assessment scores (N = 46 a)
| Day 1 | Day 8 | Day 15 | |
|---|---|---|---|
| mean ± SD | 2.7 ± 0.9 | 2.4 ± 0.9 | 2.3 ± 0.9 |
| median (range) | 3 (1 - 5) | 2 (1 - 5) | 2 (1 - 5) |
| vs. day 1b | n.s. | n.s. | |
| Patients’ distribution (N) | |||
| 1. Very satisfied | 2 | 5 | 9 |
| 2. Satisfied | 18 | 24 | 19 |
| 3. Neither | 20 | 12 | 14 |
| 4. Dissatisfied | 4 | 4 | 3 |
| 5. Very dissatisfied | 2 | 1 | 1 |
aThree patients were excluded from analysis because of missing data throughout all the three observation points.
bPost-hoc nonparametric multiple comparison analysis was performed using Steel method after significant difference in assessment scores across multiple points was detected by Freidman test (p < 0.01).
n.s.; not significant (p > 0.05).
SD; standard deviation.
Oxycodone doses on day 1 differed from 10 to 20 mg/day. Fentanyl matrix patch dose remained 12.5 μg/h in 44 patients (90%) on day 8 and 38 patients (78%) on day 15, and increased up to more than 25 μg/h in 10 patients (20%) during the study period (Table 3). Six, eight and six patients felt pain with NRS ≥ 4 at day 1, 8 and 15, respectively. Among them, one, two and two patients were under outpatient care at day 1, 8 and 15, respectively. There was no significant difference between 3 measurement days in pain intensity, rescue dose of oxycodone and adverse effects except sleepiness (Friedman test, p = 0.01) (Tables 4 and and55 and Additional file 1: Table S1).
Table 3
Dose of oral oxycodone and transdermal fentanyl matrix patch at day 0, 8 and 15 (N = 49)
| Day 1 | Day 8 | Day 15 | ||||
|---|---|---|---|---|---|---|
| Opioids | Oral oxycodone | Fentanyl patch | Fentanyl patch | |||
| Dose | ||||||
| Mean ± SD | 14.7 ± 4.1 mg/day | 13.7 ± 4.8 μg/h | 15.5 ± 6.0 μg/h | |||
| Patients’ distribution (N) | 10 mg/day | 18 | 12.5 μg/h | 44 | 12.5 μg/h | 38 |
| 15 mg/day | 16 | 18.8 μg/h* | 1 | 18.8 μg/h* | 1 | |
| 20 mg/day | 15 | 25.0 μg/h | 3 | 25.0 μg/h | 9 | |
| 37.5 μg/h | 1 | 37.5 μg/h | 1 | |||
All patients were switched from oral oxycodone to 12.5 μg/h of fentanyl matrix patch at day 1.
*Half-side application procedure of fentanyl matrix patch was not defined in our protocol.
SD; standard deviation.
Table 4
Change of numeric rating scale (NRS) of pain intensity and use of rescue immediate-release oxycodone
| Day 1 | Day 8 | Day 15 | p-valuea |
|---|---|---|---|
| NRS pain intensity (N = 49) | 0.15 | ||
| Mean ± SD | 2.2 ± 1.4 | 2.1 ± 1.5 | 1.9 ± 1.4 |
| Median (range) | 2 (0 - 6) | 2 (0 - 7) | 2 (0 - 6) |
| NRS 0 – 3 / ≥ 4 | 43/6 | 41/8 | 43/6 |
| Immediate-release oxycodone (mg / day) (N = 48)b | 0.36 | ||
| Mean ± SD | 1.9 ± 2.2 | 2.7 ± 3.5 | 2.2 ± 2.7 |
| Median (range) | 2.5 (0 - 10) | 2.5 (0 - 15) | 1.75 (0 - 10) |
SD; standard deviation.
aFriedman test.
bOne patient was excluded from analysis of rescue use because of missing data throughout all the three observation points. The remaining 48 patients completed data collection.
Fentanyl To Morphine Equivalent
Table 5
| N | Day 1 | Day 8 | Day 15 | p-valuea |
|---|---|---|---|---|
| Sleepiness | 45 | 0.004 | ||
| Mean ± SD | 1.2 ± 0.7 | 0.9 ± 0.6 | 0.73 ± 0.8 | |
| Median (range) | 1 (0 - 3) | 1 (0 - 2) | 1 (0 - 3) | |
| vs. day 1b | n.s. | <0.05 | ||
| Nausea | 49 | 0.87 | ||
| Mean ± SD | 0.4 ± 0.8 | 0.4 ± 0.7 | 0.3 ± 0.6 | |
| Median (range) | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 2) | |
| Vomit | 49 | 0.66 | ||
| Mean ± SD | 0.1 ± 0.6 | 0.2 ± 0.6 | 0.1 ± 0.5 | |
| Median (range) | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 2) | |
| Constipation | 48 | 0.08 | ||
| Mean ± SD | 0.5 ± 0.7 | 0.3 ± 0.5 | 0.3 ± 0.5 | |
| median (range) | 0 (0 - 3) | 0 (0 - 2) | 0 (0 - 2) | |
| Defecation number (/ day) | 49 | 0.85 | ||
| Mean ± SD | 1.1 ± 1.0 | 1.0 ± 0.8 | 1.0 ± 0.7 | |
| Median (range) | 1 (0 - 4) | 1 (0 - 4) | 1 (0 - 4) | |
| Epworth Sleep Scale | 49 | 0.96 | ||
| Mean ± SD | 6.3 ± 4.0 | 6.1 ± 4.2 | 6.0 ± 4.2 | |
| Median (range) | 6 (0 - 16) | 5 (0 - 18) | 5 (0 - 19) |
SD; standard deviation, n.s.; not significant (p > 0.05).
aFriedman test.
bPost-hoc nonparametric multiple comparison analysis was performed using Steel method after significant difference in assessment scores across multiple points was detected by Freidman test (p = 0.01).
Discussion
This is the first study to evaluate opioid switch directly from low dose of oral oxycodone to fentanyl matrix patch in patients with malignancy-related pain.
Another Japanese study also investigated opioid switch to 12.5 μg/h of fentanyl matrix patch from various opioids. This study was different from ours in the following three points; 1) including various kinds of prior opioid; oral oxycodone < 30 mg/day (69.4%), intravenous fentanyl injection < 0.3 mg/day (1.2%) and oral, transanal or intravenous morphine (29.4%), 2) recruiting patients with various types of primary cancers, including 34% of respiratory cancer, 3) assessing patients’ global pain assessment only at day 10, during third patch application, or the day of protocol withdrawal []. We focused on switch only from oral oxycodone ≤ 20 mg/day, recruited only patients with thoracic malignancy, and compared patients’ global assessments among 3 measurement days.
The most important finding of our study was that patients’ satisfaction was improved by opioid switch from oral oxycodone to fentanyl patch. More than 80% of patients did not feel dissatisfied in the patients' global assessment scores, which met the primary endpoint. Contrast to no difference in ESS score, sleepiness was significantly improved by opioid switch. Moreover, favorable changes were conceivably noted in constipation, though the improvement in constipation did not reach statistical significance. In the four-point categorical rating scale of sleepiness (scales 0 - 3), the number of patients with scale 0 increased from 6 on day 1 up to 19 on day 15, while that with scale 1 decreased from 29 down to 20. In the assessment of constipation, all 5 patients with scale 2 or 3 on day 1 improved to scale 0 or 1 on day 15, while one patient with scale 0 on day 1 deteriorated into scale 2 on day 15 (Additional file 1: Table S1). These changes were similar to those in the study by Miyazaki et al., in which the rate of ‘very satisfied’ and ‘satisfied’ increased over time after opioid switch to fentanyl patch [].
The second important finding was that conversion rate from oral oxycodone to fentanyl patch was not uniform. In our study, various doses of oral oxycodone from 10 to 20 mg/day were switched uniformly to 12.5 μg/h of fentanyl patch. Not a few patients felt pain of NRS ≥ 4 and needed increase of fentanyl patch thereafter. The equivalent dose of fentanyl patch to oral oxycodone was not definite in our study, though an initial conversion from 10-20 mg/day of oral oxycodone to 12.5 μg/h of fentanyl patch seems safe and reasonable. Thus, we have to pay careful attention to switch from oral oxycodone to fentanyl patch for fear of insufficient pain control or overdose.
Our study included some limitations. First, our study was not blinded. A bias derived from different formulations was possible. Second, we used an unvalidated tool to assess constipation, thereby this adverse event might be either underestimated or overestimated. Third, more than half patients concomitantly received other cancer treatment such as chemotherapy or radiotherapy. Because these concomitant treatments possibly influenced cancer pain, the true analgesic power of fentanyl patch was uncertain in those patients. Considering the poor prognosis of advanced lung cancer, we could not forbid any other cancer treatments during the study period. Fourth, the day 1 at opioid switch might not be appropriate as a baseline assessment for comparison with day 8 and 15. In addition, we should have standardized the timing of diary record. Although all patients had reported pain of NRS ≤ 3 until the previous day of opioid switch, six patients suffered from pain of NRS level ≥ 4 at the day of opioid switch (on day1). Five of these 6 patients converted opioids on day1 in our hospitals, and recorded their diaries on day 1 before or at the same time as the first application of fentanyl patch. Because we did not know when the remaining one outpatient had written diaries on day 1 at home, this patient might have severer pain than usual by the confusion from oral oxycodone to fentanyl patch. Fifth, we did not grasp the precise number of ineligible patients who dropped out during oxycodone treatment due to uncontrollable pain by 20 mg/day of oral oxycodone. Thus, we failed to clarify the proportion of eligible patients for this opioid switch maneuver in all patients with thoracic malignancy-related pain requiring strong opioid. Based on the study limitations described above, we are considering a cross-over trial comparing in pain control and adverse severity between fentanyl patch and oral opioid for cancer-related pain, instead of a randomized blinded controlled trial. Our study group is still too small to conduct such a high level of trial.
Conclusion
Transdermal fentanyl matrix patch is an alternative analgesic option for a stable cancer pain in patients with thoracic malignancies.
Transdermal Fentanyl And Conversion To Mme
Competing interest
The authors declare that they have no competing interests.
Authors’ contribution
TK designed the study. SM and TK analyzed the data and wrote the manuscript. All authors accrued and managed patients, collected data, read and approve this manuscript, and agree to its submission.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Fentanyl 75 Mcg Morphine Equivalent
Supplementary Material
Additional file 1: Table S1:The patients’ distribution of adverse events.
Transdermal Fentanyl Conversion
Acknowledgements
We thank all the investigators who recruited patients in the trial; Isao Tachibana, Hiroshi Kida, Ryo Takahashi, Toshiyuki Minami, Kotaro Miyake, Masayoshi Higashiguchi (Osaka University Graduate School of Medicine), Masanari Hamaguchi, Yoshiko Takeuchi (Osaka Police Hospital).
50 Mcg Fentanyl Patches Equivalent
References
- Jost L, Roila F, Group EGW. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21(Suppl 5):v257–260. [PubMed] [Google Scholar]
- Kroenke K, Theobald D, Wu J, Loza JK, Carpenter JS, Tu W. The association of depression and pain with health-related quality of life, disability, and health care use in cancer patients. J Pain Symptom Manage. 2010;40(3):327–341.[PMC free article] [PubMed] [Google Scholar]
- Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P, Stone PC, Tassinari D, Zeppetella G. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–68. [PubMed] [Google Scholar]
- King SJ, Reid C, Forbes K, Hanks G. A systematic review of oxycodone in the management of cancer pain. Palliat Med. 2011;25(5):454–470. [PubMed] [Google Scholar]
- Reid CM, Martin RM, Sterne JA, Davies AN, Hanks GW. Oxycodone for cancer-related pain: meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(8):837–843. [PubMed] [Google Scholar]
- Riley J, Eisenberg E, Muller-Schwefe G, Drewes AM, Arendt-Nielsen L. Oxycodone: a review of its use in the management of pain. Curr Med Res Opin. 2008;24(1):175–192. [PubMed] [Google Scholar]
- Mercadante S, Porzio G, Ferrera P, Aielli F, Adile C, Ficorella C. Low doses of transdermal fentanyl in opioid-naive patients with cancer pain. Curr Med Res Opin. 2010;26(12):2765–2768. [PubMed] [Google Scholar]
- Ahmedzai S, Brooks D. Transdermal fentanyl versus sustained-release oral morphine in cancer pain: preference, efficacy, and quality of life. The TTS-Fentanyl Comparative Trial Group. J Pain Symptom Manage. 1997;13(5):254–261. [PubMed] [Google Scholar]
- Allan L, Hays H, Jensen NH, de Waroux BL, Bolt M, Donald R, Kalso E. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ. 2001;322(7295):1154–1158.[PMC free article] [PubMed] [Google Scholar]
- Donner B, Zenz M, Tryba M, Strumpf M. Direct conversion from oral morphine to transdermal fentanyl: a multicenter study in patients with cancer pain. Pain. 1996;64(3):527–534. [PubMed] [Google Scholar]
- Kress HG, Von der Laage D, Hoerauf KH, Nolte T, Heiskanen T, Petersen R, Lundorff L, Sabatowski R, Krenn H, Rosland JH, Saedder EA, Jensen NH. A randomized, open, parallel group, multicenter trial to investigate analgesic efficacy and safety of a new transdermal fentanyl patch compared to standard opioid treatment in cancer pain. J Pain Symptom Manage. 2008;36(3):268–279. [PubMed] [Google Scholar]
- Radbruch L, Sabatowski R, Loick G, Kulbe C, Kasper M, Grond S, Lehmann KA. Constipation and the use of laxatives: a comparison between transdermal fentanyl and oral morphine. Palliat Med. 2000;14(2):111–119. [PubMed] [Google Scholar]
- Hadley G, Derry S, Moore RA, Wiffen PJ. Transdermal fentanyl for cancer pain. Cochrane Database Syst Rev. 2013;10:CD010270.[PMC free article] [PubMed] [Google Scholar]
- Tassinari D, Sartori S, Tamburini E, Scarpi E, Tombesi P, Santelmo C, Maltoni M. Transdermal fentanyl as a front-line approach to moderate-severe pain: a meta-analysis of randomized clinical trials. J Palliat Care. 2009;25(3):172–180. [PubMed] [Google Scholar]
- Tassinari D, Drudi F, Rosati M, Maltoni M. Transdermal opioids as front line treatment of moderate to severe cancer pain: a systemic review. Palliat Med. 2011;25(5):478–487. [PubMed] [Google Scholar]
- Wirz S, Wittmann M, Schenk M, Schroeck A, Schaefer N, Mueller M, Standop J, Kloecker N, Nadstawek J. Gastrointestinal symptoms under opioid therapy: a prospective comparison of oral sustained-release hydromorphone, transdermal fentanyl, and transdermal buprenorphine. Eur J Pain. 2009;13(7):737–743. [PubMed] [Google Scholar]
- Mercadante S. Opioid rotation for cancer pain: rationale and clinical aspects. Cancer. 1999;86(9):1856–1866. [PubMed] [Google Scholar]
- Morita T, Takigawa C, Onishi H, Tajima T, Tani K, Matsubara T, Miyoshi I, Ikenaga M, Akechi T, Uchitomi Y. Japan Pain, Rehabilitation Palliative Medicine Psycho-Oncology Study, Group. Opioid rotation from morphine to fentanyl in delirious cancer patients: an open-label trial. J Pain Symptom Manage. 2005;30(1):96–103. [PubMed] [Google Scholar]
- Takakuwa O, Oguri T, Maeno K, Yokoyama M, Hijikata H, Uemura T, Ozasa H, Ohkubo H, Miyazaki M, Niimi A. Analgesic effect of switching from oral opioids to a once-a-day fentanyl citrate transdermal patch in patients with lung cancer. Am J Hosp Palliat Care. 2013;30(7):726–729. [PubMed] [Google Scholar]
- Miyazaki T, Hanaoka K, Namiki A, Ogawa S, Kitajima T, Hosokawa T, Ishida T, Nogami S, Mashimo S. Efficacy, safety and pharmacokinetic study of a novel fentanyl-containing matrix transdermal patch system in Japanese patients with cancer pain. Clin Drug Investig. 2008;28(5):313–325. [PubMed] [Google Scholar]